MORGANTOWN — With the first batches of the Pfizer COVID-19 vaccine expected to arise in the middle of this month, some people are over-optimistically thinking full vaccination and herd immunity is right around the corner.
Gov. Jim Justice on Friday expressed the hope all West Virginians could be vaccinated by mid-March. It may, in fact, take longer.
The Dominion Post spoke with COVID-19 Czar Clay Marsh about how the vaccination process could unfold. We also draw from Marsh and various news and health media reports for a look at how the vaccine works.
Pifzer and Moderna are both seeking emergency use authorization from the FDA to distribute their vaccines. This authorization allows them to get doses out quickly. Full FDA authorization would come when we understand the long-term effectiveness of the vaccines and how often booster shots will be needed.
Pfizer, Marsh said, is about a week ahead of Moderna and should get FDA OK first. Jutice’s COVID team anticipates the first Pfizer shipment could arrive Dec. 15, with Moderna’s first shipment maybe a week later. The United Kingdom has already granted emergency authorization to Pfizer and will start vaccinating people this week.
Shipments will go out on a pro-rated on population basis, Marsh said. Justice and Adjutant General James Hoyer said Friday the first shipment may be close to 20,000 doses with shipments of about 21,000 – Pfizer and Moderna combined – to follow in subsequent weeks.
Marsh said, “We believe, then, as time goes forward, the Pfizer vaccine will keep coming and the doses will grow over time as manufacturing goes up. The Moderna vaccine will keep coming and doses will grow over time.”
Both vaccines require two doses for full effectiveness, Pfizer’s 21 days apart, Moderna’s 28 days. Then it takes another two to three weeks to reach full potency.
“So even if we started in December,” Marsh said, “the first group of people would not be fully vaccinated until mid-January and by the time their immune system sort of maximally produces the antibodies, the response to the vaccine, we’re talking probably in February for the first group of people to get the maximum benefit.”
Based on CDC guidance, Justice unveiled on Friday how the first batches will go out.
The vaccine will be administered in phases as shipments come in. In Phase 1, health care workers will come first, followed by long-term care facility residents and staff, then community infrastructure and emergency response personnel, public health officials and first responders. This covers more than 100,000 people.
The plan from there for the general public is to scale to five hubs – in Monongalia, Berkeley, Cabell, Kanawha and Greenbrier counties – and then out to 250 administration sites.
While both are similar, Marsh said, Pfizer’s is less stable has to be around -94 degrees Fahrenheit and transported on dry ice. When it’s reconstituted into individual doses it has six-hour refrigerator shelf life. So there won’t be the luxury to ship it to every doctor’s office and a more central distribution approach is likely. Moderna’s is more forgiving, being stored at only -4 Fahrenheit. SO Pfizer’s will be best suited in areas with a higher density population.
Asked for a ballpark on how soon the whole state might be vaccinated, Marsh said, “It’s hard to know how soon we’ll see accelerated manufacturing of doses and delivery of more doses.” The very earliest could be mid-2021 with a good guess being third quarter 2021.
An extended possibility is fourth quarter 2021 into 2022. “I don’t think that will happen but it’s possible.”
Pfizer has said it has the manufacturing capacity to produce up to 50 million vaccine doses globally in 2020 and up to 1.3 billion doses by the end of 2021.
Other vaccine contenders
Along with Pfizer and its partner BioNTEch, and Moderna, several other drug makers signed a pledge committing to “developing and testing potential vaccines for COVID-19 in accordance with high ethical standards and sound scientific principles.”
The others were AstraZeneca, Johnson & Johnson, Sanofi and partner GlaxoSmithKline, Merck and Novavax.
AstraZeneca and Johnson & Johnson are leading the next wave, Marsh said. Their vaccines operate on a different strategy and may not be quite as effective but are still very good. AstraZeneca’s is roughly 70% effective. “And that vaccine generally would be a lot cheaper, so this will be really important for the developing world as well, to get the vaccine to more people around the world.”
Both companies are behind because the vaccines produced a side effect that stopped human trials temporarily. Marsh thinks it may be sometime into 2021 fro AstraZeneca to get FDA approval, and the same in other countries.”
How they work
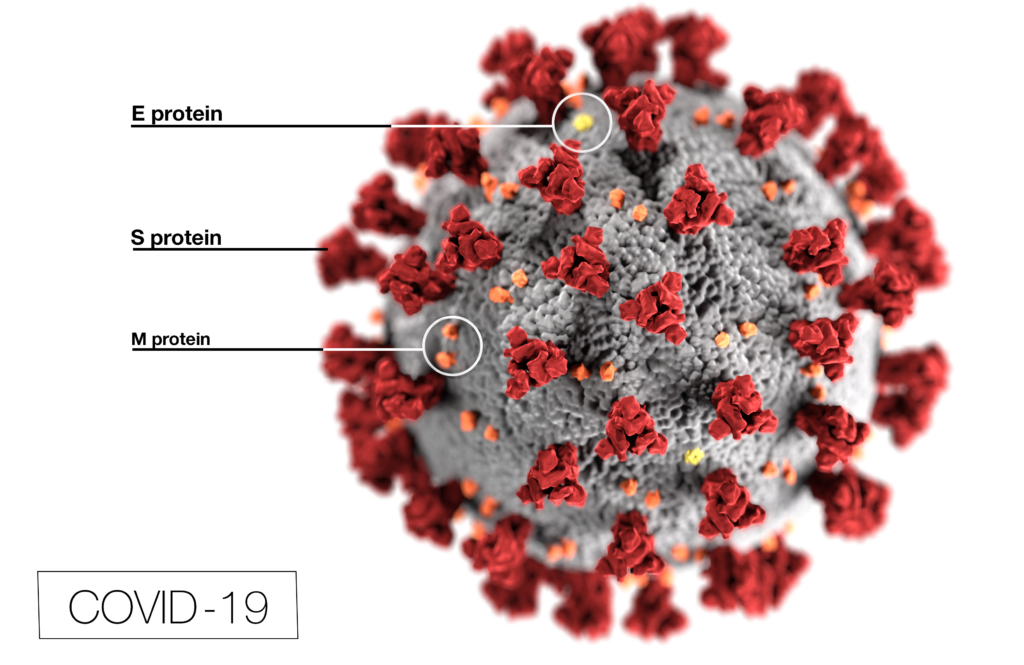
Pictures of the COVID-1p virus show it to be a ball covered in spiky mushroom or suction-cup-looking projections. Those projections are called spike proteins.
Pfizer and Moderna separately developed a groundbreaking approach to their vaccines, Marsh said.
As Pfizer explains it, their vaccines are called mRNA vaccines. As with other vaccines we’re familiar with, they are not made up of the actual pathogen, meaning that they don’t contain weakened, dead, or noninfectious parts of a virus or bacterium.
The COVID vaccines instead contain genetic information about the virus, which uses the spike protein to connect with and attack our cells.
The vaccines use a man-made spike protein, Pfizer explained, to train the body to identify and block the virus spike protein interaction or to recognize a virus-infected cell. Then, when the real virus enters the body, the immune system can recognize and destroy the virus by summoning its defenses.
The Pfizer and Moderna vaccines put the man-made gene sequence inside fat globules, called lipozones, that can bind to cells and deliver the vaccine inside cells.
AstraZeneca and Johnson & Johnson, Marsh said, use a different kind of virus to package the spike protein. It’s possible that the reactions that halted their trials for a time were due to the packaging virus.
Side effects
Pfizer and Moderna have reported that their vaccines are safe and overall well tolerated.
Moderna vaccine side effects include injection site pain, fatigue, muscle aches, joint pain, headache and low-grade fever. Pfizer’s include injection site pain, fatigue, headache and low-grade fever.
Both companies said low-grade fever of 102-104 degrees occurred in less than 2% of recipients.
Side effects can last a day or two for both.
The bottom line
“The vaccines are not the big deal,” Marsh said. “Vaccination of people, that’s the big deal. We want to make sure that people who have access to the vaccines feel safe and comfortable in taking it and their family taking it.
“It is really when all of us become vaccinated and we all become that link in the chain that stops the pread is when West Virginia will be able to rise from the dark clouds that COVID has generated over us and get back to all the things that we value.”
Tweet David Beard@dbeardtdp Email dbeard@dominionpost.com




